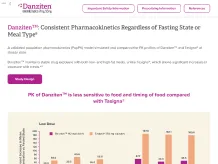
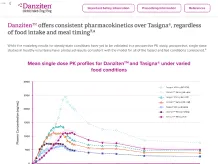
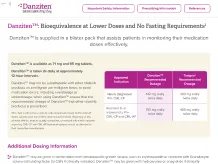
Danziten™: Bioequivalence at Lower Doses and No Fasting Requirements2
Danziten™ is supplied in a blister pack that assists patients in monitoring their medication doses effectively.
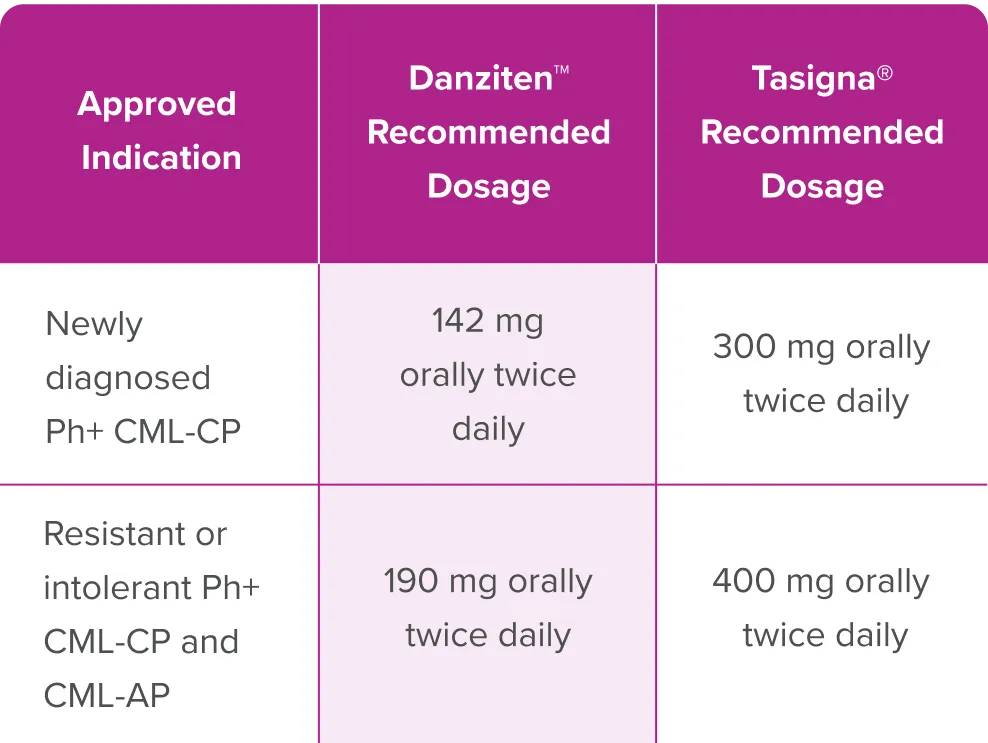
Danziten™ is available as 71 mg and 95 mg tablets.
Danziten™ is taken 2x daily at approximately 12-hour intervals.
Danziten™ may not be substitutable with other nilotinib products on a milligram per milligram basis; to avoid medication errors, including overdosage or underdosage, when using Danziten™ ensure that the recommended dosage of Danziten™ (not other nilotinib products) is prescribed.
NCCN Guidelines for CML state that nilotinib products available in different formulations, dosage form, and strengths are not interchangeable.1
This chart is offered as a side-by-side comparison based on the product labels. Azurity does not confirm other products’ labels. Reporting of any adverse effects, product quality complaints, or medical information request regarding CML-CP and CML-AP treatment options should be directed to their respective manufacturer.
NCCN Guidelines contain other treatment regimens. NCCN makes no warranties of any kind whatsoever regarding their content, use, or application and disclaims any responsibility for their application or use in any way.
Recommendations for Switching From Tasigna® to Danziten™
Additional Dosing Information
- Danziten™ may be given in combination with hematopoietic growth factors, such as erythropoietin or replaced with Granulocyte colony-stimulating factor (G-CSF) if clinically indicated. Danziten™ may be given with hydroxyurea or anagrelide if clinically indicated
- Reduce starting dose of Danziten™ in patients with hepatic impairment
- Withhold or reduce Danziten™ dosage for hematological toxicities (neutropenia, thrombocytopenia) that are not related to underlying leukemia
- For non-hematolgic laboratory abnormalities (Grade ≥3) such as elevated serum lipase, amylase, bilirubin, or hepatic transaminases, withhold Danziten™ and monitor levels, resume treatment at 190 mg once daily when serum levels return to Grade ≤1
- Advise patients to swallow the tablets whole with water and not to cut, crush, or chew the tablets
- Advise patients to take Danziten™ with or without food. Patients should not consume grapefruit products and other foods that are known to inhibit CYP3A4 at any time during Danziten™ treatment
- If the patient missed a dose of Danziten™, the patient should take the next scheduled dose at its regular time. The patient should not take 2 doses at the same time